Precision engineering has revolutionized the field of medicine, particularly in joint replacements. Among the companies that have contributed to this advancement is Exactech. They are a prominent medical device manufacturer that specializes in implants and technology for joint replacements.
However, despite the promise of precision engineering, Exactech has faced significant challenges due to product recalls surrounding its hip replacement technology. This article discusses the role of precision engineering in medicine, the complexities of hip replacement technology, and the impact of product recalls.
The Promise of Precision Engineering

Precision engineering has become a driving force behind medical advancements, offering the potential for customized and optimal solutions for patients. In joint replacements, this technology allows for tailored implants that fit an individual’s anatomy more accurately.
This improves overall outcomes and patient satisfaction. By utilizing sophisticated imaging techniques and computer-assisted design (CAD), medical professionals can now create hip replacements that mimic natural joint movement and function.
According to Future Market Insights, the adoption of CAD software in the healthcare sector has brought about a significant transformation in the industry. Developers are now directing their efforts toward enhancing the functionalities and capabilities of medical equipment, marking a revolutionary shift in the field.
Understanding Hip Replacement Technology
Hip replacement surgery is a common procedure designed to alleviate pain. It can also improve joint function for individuals suffering from severe hip conditions like osteoarthritis and rheumatoid arthritis.
The typical components of a hip replacement include the stem, hip cup, liner, and ball. Exactech’s hip replacement technology aimed to leverage precision engineering to create durable and long-lasting implants. However, some products have faced challenges with premature wear and degradation due to packaging errors. This has led to product recalls and the need for corrective revision surgeries.
The Impact of Product Recalls on Patients
The recall of more than 140,000 Exactech knee, hip, and ankle implants due to packaging mistakes brings attention to the possible hazards linked with precision-engineered medical devices. As cited in a report by ConsumerSafety, the packaging error resulted in the plastic insert component being exposed to oxygen, which can initiate oxidation. As indicated by Exactech, this oxidation can lead to premature degradation of the plastic insert.
As a result, patients who received these implants face the burden of potential complications, pain, and the necessity for additional surgeries. Apart from the physical ramifications, patients must endure emotional stress and uncertainty about their health and future mobility.
As the medical industry strives for innovation and precision, such recalls underscore the need for rigorous testing. They also highlight the need for quality control and long-term monitoring of medical devices to ensure patient safety.
The Exactech Lawsuits
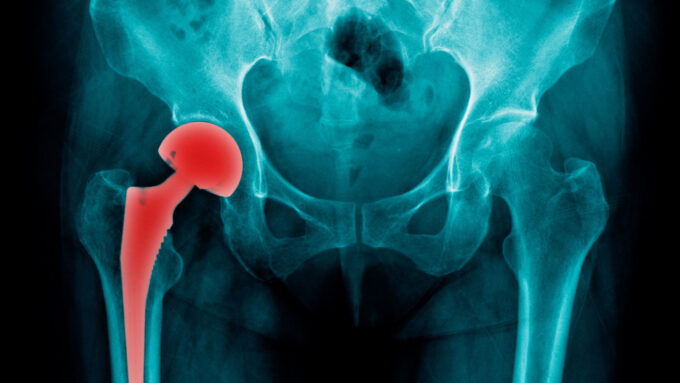
The Exactech hip replacement recall has led to numerous lawsuits against the company. Patients affected by faulty implants and their legal representatives are seeking accountability. They seek compensation for the pain, suffering, and additional medical expenses incurred due to the defective products. These litigations emphasize the importance of transparency, accountability, and patient rights in the medical device industry.
As of July 2024, ConsumerNotice.org reports that there are currently 395 pending lawsuits in New York multidistrict litigation related to Exactech. However, as of that date, there have been no trials or approved settlements in these lawsuits. Lawyers involved in the litigation estimate that a potential settlement for the Exactech recall lawsuits could range from $100,000 to $300,000.
According to TorHoerman Law, it’s important to note that the final settlement amount for each claimant could vary depending on the specific injuries claimed in their individual cases. This could potentially result in either a smaller or larger settlement.
Emphasizing Patient-Centric Approaches
The litigation surrounding Exactech’s hip replacement recalls highlights the need for a patient-centric approach to medical device development. Patient safety and well-being should always be at the forefront of any medical innovation.
By prioritizing rigorous testing, transparent communication, and ongoing monitoring of medical devices’ performance, the industry can foster trust between manufacturers, medical professionals, and patients.
Examining Exactech’s Response and Corrective Measures
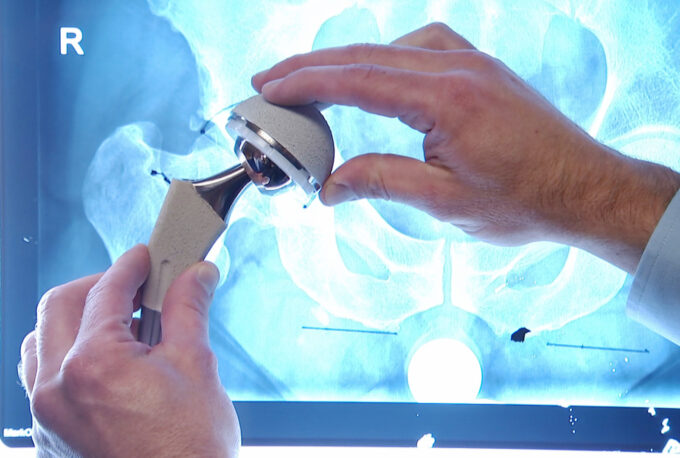
An integral part of any product recall scenario is the manufacturer’s reaction and the subsequent steps taken to amend the situation. In the case of Exactech, an understanding of their corrective actions could offer valuable insight into their commitment to patient safety. Their response may encompass significant enhancements in their manufacturing procedures, stringent quality control protocols, and effective patient communication mechanisms.
The strategic revision of these key areas demonstrates their dedication towards rectifying errors and establishing a safer future for their products. By showcasing this, readers gain a nuanced understanding of Exactech’s proactive approach towards addressing the recall situation.
Unveiling Patient Support and Rehabilitation Efforts

The ramifications of product recalls extend well beyond the immediate physical and emotional distress experienced by patients. Therefore, it is crucial to discuss the comprehensive support and rehabilitation programs that have been put in place to aid those affected by these recalls.
Such initiatives could range from helping patients navigate the complexity of living with faulty implants, ensuring access to necessary medical treatments for corrective surgeries, to offering counseling services to tackle psychological challenges. By spotlighting these programs, readers are informed about the importance of holistic patient care during these difficult times, illuminating how support goes beyond just the medical aspect of treatment.
When navigating through product recalls, it’s paramount to consider the full range of responses, corrective actions, and patient support services. Understanding these facets not only provides a clearer picture of the current situation but also empowers readers to make informed decisions and develop a greater appreciation for the comprehensive efforts undertaken during such incidents. This is not a matter that should preoccupy only the manufacturer but broader layers of society too.
Ending Note
The case of Exactech’s hip replacement technology and the subsequent product recalls demonstrate the potential risks and complexities associated with precision-engineered medical devices. While precision engineering holds the promise of customized solutions, it also demands rigorous testing and quality control to ensure patient safety.
The impact of product recalls on affected patients goes beyond physical complications, causing emotional distress and necessitating litigation seeking accountability. These incidents underscore the importance of prioritizing a patient-centric approach in medical device development.
These incidents also emphasize the importance of transparency, ongoing monitoring, and stringent safety measures to foster trust between manufacturers, medical professionals, and patients. Moving forward, the industry must learn from these experiences to enhance patient safety and well-being in the pursuit of medical innovation.